Pancreatic Cancer - Understanding Clinical Trials
*Please note: This slide set represents a visual interpretation and is not intended to provide, nor substitute as, medical and/or clinical advice.
Clinical trials are research studies that evaluate new ways to improve treatments and quality of life for people with diseases like pancreatic cancer.
Clinical trials are an option for all patients to consider and play an important role in improving healthcare
Results from clinical trials help develop new drugs, new diagnostic tools, and new clinical procedures.
Clinical trials can also spur new questions for research, which lead to new discoveries over time and help us to better understand diseases like pancreatic cancer.
All the drugs and tools we have today were made possible because patients volunteered for clinical trials.
Every clinical trial has what are called “eligibility criteria”.
For example, a patient may need to have a certain medical condition, or gender, or other specifics to meet the criteria of participating in the clinical trial. Otherwise they are not eligible to join that clinical trial and could seek another trial that better fits their situation.
Patients who want to volunteer need to understand the potential benefits and risks of participation in a clinical trial.
Patients may personally
benefit
from receiving new therapies or procedures. They receive close supervision from clinical staff. Sometimes the cost of the new drugs or testing is free or waived. Participants may also be part of a research breakthrough that helps thousands of future patients.
Patients face the
risk
that they may not benefit from the new treatment. Additionally, participating in a clinical trial requires commitment. Trials can be lengthy or demanding, and may require extra treatments, extra time in the clinic or tests or costs that are not part of standard care. It is possible, if the study is randomized, that they may be part of the control group that does not receive the new drug being studied.
“Informed consent” is a process that helps patients make an informed decision about whether to join a clinical trial.
An “informed consent document” will be provided that explains the purpose, procedures, and potential benefits and risks of the trial.
There will also be thorough discussions between the doctor and patient so that all questions can be clearly answered.
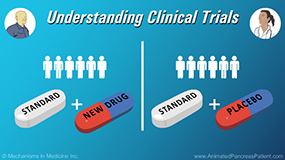
In late-stage clinical trials, participants are generally split into 2 groups at the beginning of the study.
One group receives the standard treatment plus the new treatment. This group is called the “Treatment Group”.
The other group receives the standard treatment only, and does not receive the new treatment. This group is known as the “Control Group”.
A computer is used to randomly assign the patient to either the Treatment Group or the Control Group.
Randomization helps to prevent bias because neither the patient nor the doctor can choose which treatment group a patient is assigned to.
Some clinical trials may want to study the effectiveness of adding a new treatment to a standard treatment to see what works better.
In these studies, half the patients will receive the standard treatment plus the new treatment, and the other half will receive the standard treatment plus a “placebo”.
A “placebo” is a treatment that looks identical to the new treatment, but contains no active ingredient.
These types of studies are often called “blinded", meaning that one or more parties involved in the trial do not know which patients have been assigned to which treatment.
“Single-blinded” means that only
one
party (either the researchers or the patients) knows which patients have been assigned to which treatment.
“Double-blinded” means that both the researchers and the patients do not know which patients have been assigned to which treatment.
The strongest, most reliable results come from clinical trials in which the treatment is double-blind, the patients are randomized, and the study has a control group.
This is called a “Double-blinded Randomized Controlled Clinical Trial”.
Clinical trials are carefully monitored to protect patients, and several layers of review committees are involved to protect patients from any possible harm.
Patients can leave a clinical trial at any stage. However, they should discuss this with their doctor before exiting, to ensure the safest procedures are followed.
There are 4 different phases of clinical trials.
Safety is emphasized in each phase. If a trial does not have a positive or safe outcome in Phase 1, then it will not be allowed to proceed to a Phase 2 trial, and so on.
Before a Phase 1 trial begins, there is a Phase 0, where the new treatment has already been found to be safe in animals or healthy human volunteers.
In Phase 1 clinical trials, a new treatment is tested in a small group of participants for the first time to determine a safe dose and see how the treatment affects the body.
Phase 2 is conducted with a larger patient group than the previous phase and studies how well a new treatment or combination of treatments works for a certain type of cancer, like pancreatic cancer.
At this stage the eligibility criteria become more restrictive.
Phase 2 studies can be either randomized or non-randomized.
Phase 3 trials are conducted in an even larger group of participants. This phase involves a randomized design, comparing the new treatment to the standard treatment.
If a Phase 3 clinical trial is positive, typically the new treatment will be approved and licensed for use in the United States by the Food and Drug Administration (or FDA).
After the treatment has been approved, Phase 4 clinical trials involve monitoring to continue to assess safety, effectiveness, and optimal use.
Clinical trials play an important role in moving science forward and can be a hopeful option for many patients with pancreatic cancer.
To learn more about clinical trials and how to participate in one, please talk to your doctor.
You can also visit The National Pancreas Foundation’s Clinical Trial Resource Centre at www.PancreasFoundation.org and the www.ClinicalTrials.gov website to learn more.