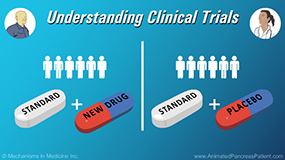
Physicians explain how clinical trials work and the different types of clinical trials and stages – from early stage to late stage. In a clinical trial, there is an individual called the principle investigator, who has primary responsibility for the conduct of the trial, and he or she will work with a team, usually a research nurse and several study coordinators and data managers. The trial is overseen by an institutional review board (IRB) and other committees that govern the scientific merits of the study, and patient welfare and safety. A clinical trial has to be done under rigorous oversight and controls, so it usually means that patients receive treatment in a clinical trial center and not always with their local community oncologist.
-
Share with family and friends:
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about pancreatic diseases which will help other patients, caregivers and families.
Other Sections in this Module: