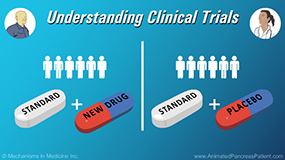
Physicians explain what randomization, standard of care arm and experimental arm mean in clinical trial study design. Randomization is where participants are assigned by chance to separate groups that compare different treatments, and neither the participant, nor the doctor, nor the medical team can choose which group the participant is assigned to. Typically this is assigned by computer allocation. They also explain that to help support the development of new drugs there needs to be a reference, and that reference is called a standard approach or ‘standard of care.’ Participants may be assigned to the standard of care arm of a study where they receive the standard of care treatment plus placebo, or to the experimental arm where they receive the standard of care plus the new treatment of interest. If a study design is ‘blinded’ it means that one or more parties involved in the trial (such as the participant, or researchers, or sometimes both) do not know which study arm the participant is assigned to.
-
Share with family and friends:
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about pancreatic diseases which will help other patients, caregivers and families.
Other Sections in this Module: