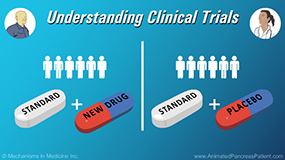
Physicians discuss what a “placebo” is in a clinical trial, and if is it always used. Placebos can have some real impacts on a clinical trial. Researchers know that there is an effect from the trial, but they need to isolate that effect to make sure it is really the medicine or device that is causing that effect. In some clinical trials, doctors want to learn if adding a new drug to the standard therapy makes it work better. In these studies, some patients get the standard drug(s) and the new one being tested, while other patients get the standard drug(s) and a placebo. But none of the patients would get only a placebo. The placebo may look like medicine but it has no active ingredient in it. This clinical trial context is typically “blinded”, meaning neither the doctors nor the patients know which group they are in. This ensures there are no doctor or patient biases that impact the outcomes of the clinical trial.
-
Share with family and friends:
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about pancreatic diseases which will help other patients, caregivers and families.
Other Sections in this Module: