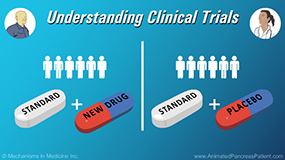
Physicians describe the safeguards that are put in place to protect patients in clinical trials. At the beginning, the initial idea for the study undergoes a series of reviews to make sure the study idea is appropriate, that patients will be safe, that the right drugs are being used, and the right end points are going to be answered. A big part of the safeguard process is institutional review board (IRB) oversight. An IRB is a committee that reviews the trial to ensure the science and safety aspects for patient care. Furthermore, the principal investigator has an ethical and professional obligation to both the patient and to the study. In addition, on a national level, there are federal safeguards in place that oversee clinical trials. For example, periodically, audits can be done at institutions of clinical trials to make sure there isn't an important safety signal being missed to make sure that the data is reported accurately and in a timely fashion.
-
Share with family and friends:
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about pancreatic diseases which will help other patients, caregivers and families.
Other Sections in this Module: