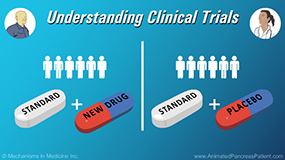
Physicians discuss what happens if a patient changes their mind and wants to leave a clinical trial. A patient has every right to choose to stop participating in a clinical trial at whatever time point they want, even if they don't meet formal criteria for having to be taken off the trial. A patient will be removed from a study if the treatment is not working, if their cancer is continuing to grow, if researchers observe an unacceptable level of potentially serious side effects that may be life-threatening, or if the clinical trial is affecting their quality of life too much. There are also occasions where a patient finds that a clinical trial is just too time-intensive. It’s always important for a patient to talk with the researchers about their reasons for leaving the trial and to make sure that everything is done in the safest manner possible.
-
Share with family and friends:
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about pancreatic diseases which will help other patients, caregivers and families.
Other Sections in this Module: