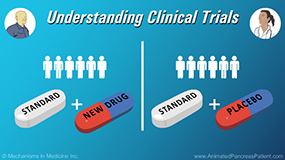
Physicians explain what “informed consent” is in a clinical trial. Informed consent is the process of giving clinical trial participants all of the facts about a trial. Informed consent is greatly important because patients cannot participate in a study without their clear understanding of everything involved. Informed consent means that the patient is making an informed decision on their own, and understands all the risks and benefits for participating in a study. There is a verbal conversation that goes along with this and also a written document. The informed consent document is vetted by an institutional review board (IRB) to make sure it’s not missing anything. Both the physician and the patient will sign the consent form, and then the next step is screening for eligibility and, if the patient qualifies, moving forward with the clinical trial.
-
Share with family and friends:
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about pancreatic diseases which will help other patients, caregivers and families.
Other Sections in this Module: